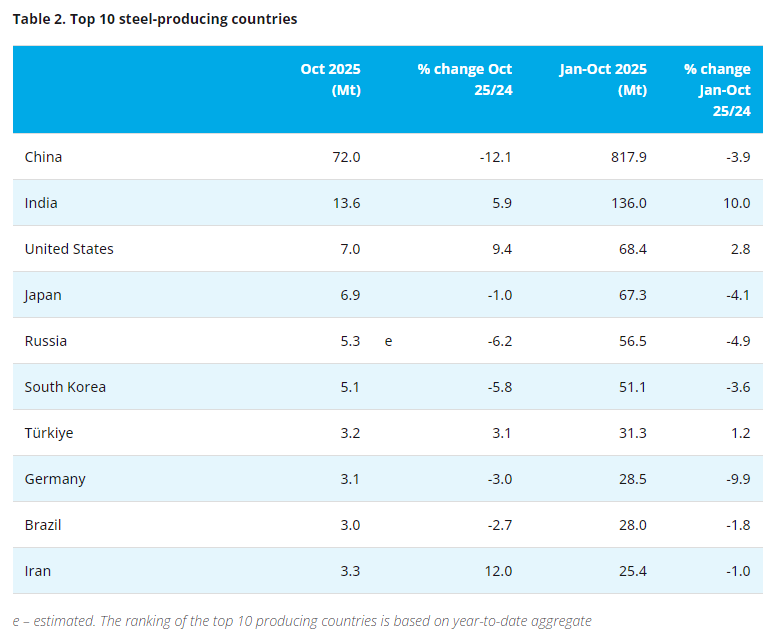
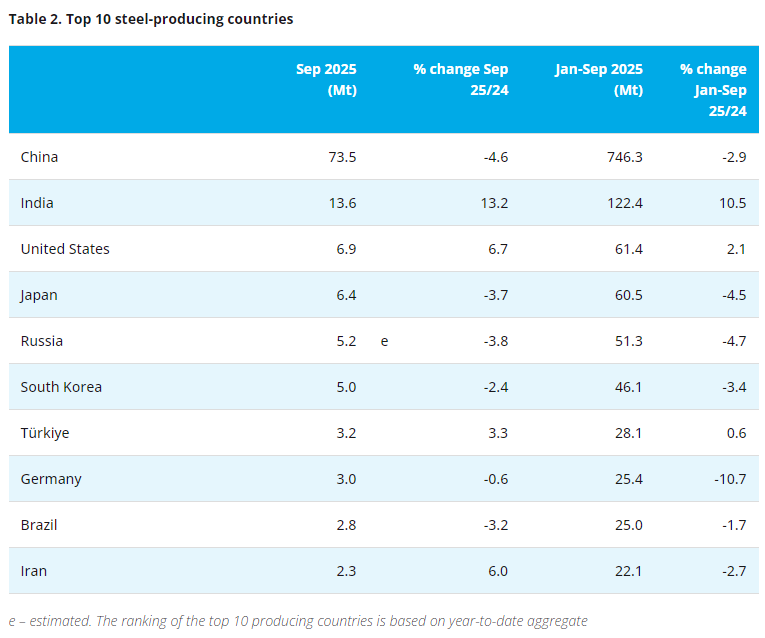
[www.ferro-alloys.com]An international team of scientists that includes researchers at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) has revealed how interactions between electrons and ions can slow down the performance of a material considered key to the next generation of batteries.
A scanning electron microscopy image of vanadium pentoxide nanowires. The inset shows a ball-and-stick model of vanadium pentoxide’s atomic structure before and after inserting lithium ions. (Credit: Texas A&M University)
As the appetite grows for more efficient vehicles and mobile devices based on cleaner, renewable energy sources, so does the demand for equally efficient, lightweight and energy-dense batteries that pack more punch, last longer and charge or discharge more quickly. The compound vanadium pentoxide, with its layered atomic structure, has grabbed the spotlight as a potential nanostructured material for state-of-the-art lithium-ion batteries because it can provide a greater surface area for the arrival and insertion of lithium ions. That quality makes vanadium pentoxide a good candidate as a cathode, the part of a battery where electrons and lithium ions enter.
X-ray absorption fingerprints showing different lithium concentrations in well-separated regions in a single vanadium pentoxide nanowire. (Credit: Texas A&M University)
“The speed with which electrons can enter and exit the cathode determines how much power the battery can provide and how quickly it can be recharged, both critical factors to consider in the world of mobile electronics or electrification of our automotive fleet,” said David Prendergast, staff scientist at the Molecular Foundry, a DOE Office of Science User Facility located at Berkeley Lab.
But despite vanadium pentoxide’s potential, it has yet to be widely adopted commercially because of its less-than-stellar performance when put to the test in the real world.
The new findings, published this week in the journal Nature Communications, shed light on the slowdown, showing that the flow of electrons in vanadium pentoxide nanowires gets bogged down as it interacts with lithium ions in a phenomenon known as “small polaron formation.”
The study is led by Sarbajit Banerjee, professor of chemistry at Texas A&M University and a user of Berkeley Lab’s The Molecular Foundry. Banerjee’s team worked with Prendergast and postdoctoral fellow Yufeng Liang on this discovery through a user project at the Molecular Foundry.
The Banerjee group made 2D maps of the electronic properties of synthesized vanadium pentoxide nanowires serving as a model lithium-ion cathode using Scanning Transmission X-ray Microscopy at The Canadian Light Source. They came to the Molecular Foundry to interpret their findings.
The above schematic shows different views of the same crystal structure in vanadium pentoxide. The left panel shows a typical, expanded distribution of electrons that can conduct electrical current. The right panel shows a polaron, or the localization of electron density, which slows down the movement of electrical current and ions. (Credit: Yufeng Liang/Berkeley Lab)
These nanoscale measurements provided evidence that electrons in vanadium pentoxide sit predominantly on vanadium atoms near lithium ions and induce a distortion in the surrounding crystal structure. The combination of a charged particle – the electron – and an associated local structural distortion is referred to as a “small polaron.”
“Small polarons have been proposed as the cause of the slowdown in lithium-ion transport inside cathode materials, but they had not been seen directly until now,” said Liang. “With the help of our simulation techniques, we were able to decipher the polaron information buried in the X-ray spectra.”
“The electrons, once coupled with the lithium ions, appear content to sit instead of moving freely, in essence, trapping or stranding the flow of energy,” Prendergast added.
Now that the bottleneck has been verified, the researchers said that attention can be turned to finding ways to design materials that free up the flow of electrons.
The National Science Foundation helped support the work of Banerjee, while the U.S. Department of Energy supported theoretical and computational activity at The Molecular Foundry.
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time.
- [Editor:Jiang Li Juan ]



 Save
Save Print
Print Daily News
Daily News Research
Research Magazine
Magazine Company Database
Company Database Customized Database
Customized Database Conferences
Conferences Advertisement
Advertisement Trade
Trade








Tell Us What You Think